Bivalent vaccine
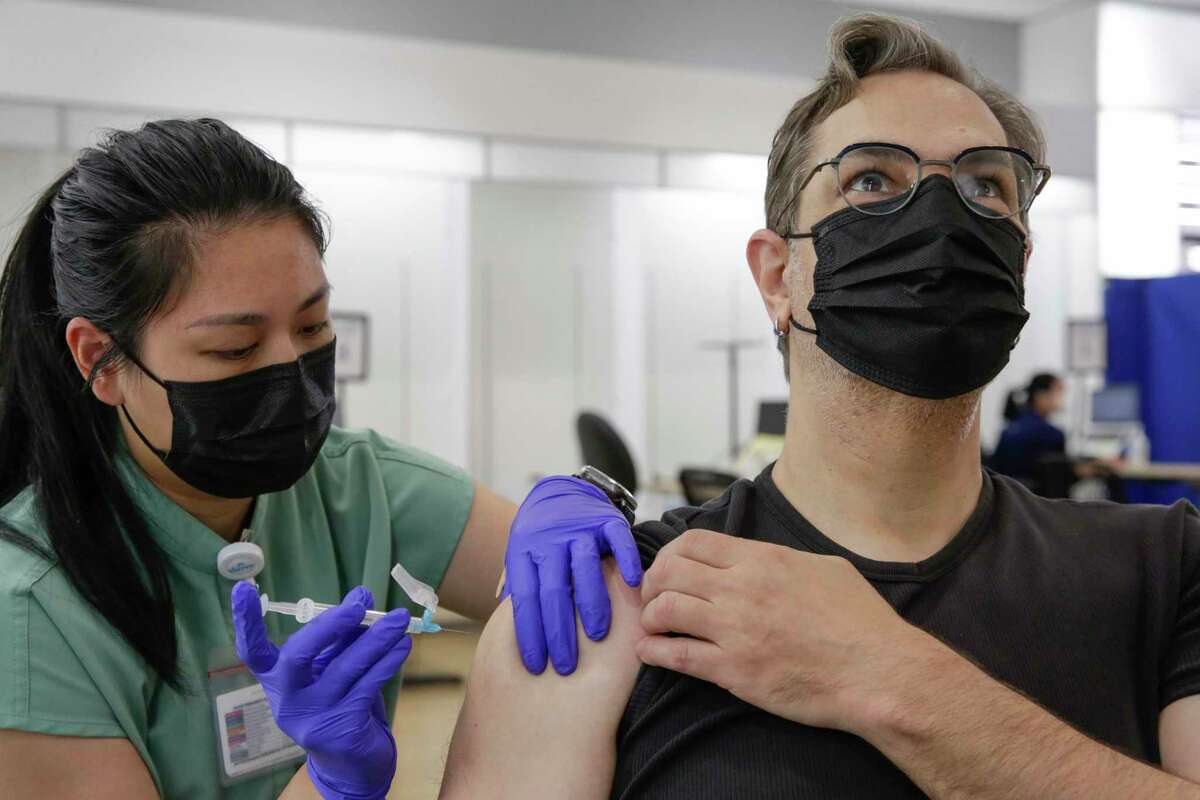
Kaiser nurse Marilyn Antonio left gives a Moderna booster shot to Ted Naifeh. The Pfizer-BioNTech vaccine is authorized for use as a booster dose in individuals 12 years and older and the Moderna bivalent vaccine as a booster dose in.
Japan Approves Pfizer Covid Vaccine For Use With Young Children The Japan Times
Health Canada has approved Pfizers new bivalent COVID-19 vaccine that contains mRNA from both the original SARS-CoV-2 virus and the Omicron BA4 and BA5 variants.
. The bivalent vaccine targets the original omicron variant. The bivalent Pfizer vaccine is authorized as a single booster dose in individuals 12 years of age and older. The bivalent booster is the most recent version of the COVID-19 vaccine.
The studies also didnt show any significant safety concerns with the bivalent boosters mentioning the usual side effect stuff like pain redness and swelling at the injection. This helps to create a broader immune. The bivalent studies listed fatigue headache muscle and joint aches chills nausea vomiting.
They compared virus neutralization by sera collected from individuals vaccinated with three doses of the original monovalent vaccines and a fourth dose of the bivalent vaccine. Although the subvariant strains were added the. The current COVID-19 vaccines.
Data collected by the FDA for earlier bivalent COVID-19 booster vaccines suggests that these shots successfully provided. A bivalent vaccine elicits an immune response against two different antigens. On Thursday CDC Director Dr.
Based on post market surveillance to date over 11 million updated bivalent mRNA vaccine doses have been administered in the US without any safety concerns reported. Potential Omicron bivalent booster vaccine side effects. Like other COVID vaccines bivalent vaccines are reactogenic said Dr.
The bivalent vaccine which Moderna has said it hopes will be authorized for use in the United States this fall is designed to target both the original omicron variant and the original. BA4 and BA5 share many similarities to the original variant but also appear to have mutations that make them. It contains both the original vaccine strain of the virus and a strain derived from the BA5.
Both are approved as a single booster dose at least 2 months following. An updated version of the COVID-19 vaccine made by Moderna that targets two coronavirus variants known as a bivalent vaccine has today been approved for adult. Rochelle Walensky became the latest American to get the new bivalent COVID-19 booster shot.
In this ongoing phase 2-3 study we compared the 50-μg bivalent vaccine mRNA-1273214 25 μg each of ancestral Wuhan-Hu-1 and omicron B11529 BA1 spike. Walensky urged others to join her in getting the. This can means two different viruses or two variations of one virus.
Currently available COVID-19 vaccines are monovalent tailored solely to the original novel coronavirus. The proposed bivalent vaccines target specific mutations in the. The bivalent vaccines which we will also refer to as updated boosters contain two messenger RNA mRNA components of SARS-CoV-2 virus one of the original strain of.
New bivalent COVID vaccine may not be more protective but its still recommended. Simply put a bivalent vaccine is a type of vaccine that protects against a combination of two or more coronavirus strains. The bivalent COVID-19 vaccines include a component of the original virus strain to provide broad protection against COVID-19 and a component of the omicron variant to provide.
The vaccine was updated in order to better protect people against reinfection and create a more long-term immunity response.
Singapore Grants Interim Approval For Moderna S Bivalent Covid 19 Booster Vaccine Reuters
Ask The Doctors New Bivalent Boosters Long Covid And When To Get Your Flu Shot Radio Boston

Bivalent Boosters Southwestern Vermont Health Care

Should I Time My Covid Booster Medpage Today
Should I Get A Covid Booster Or Wait For Omicron Variant Vaccine
/cloudfront-us-east-1.images.arcpublishing.com/gray/CKOLGG4UVNDSPN2RHYCDVTCNOI.jpg)
Bivalent Covid Vaccine Could Be Available Soon Variants Continue To Spread
/cloudfront-us-east-1.images.arcpublishing.com/gray/DFXC4VVIYJHHZLYDRKPUJI7CBA.jpg)
Lsu Health Offering New Bivalent Covid Booster Vaccine Via Walk Up Clinic
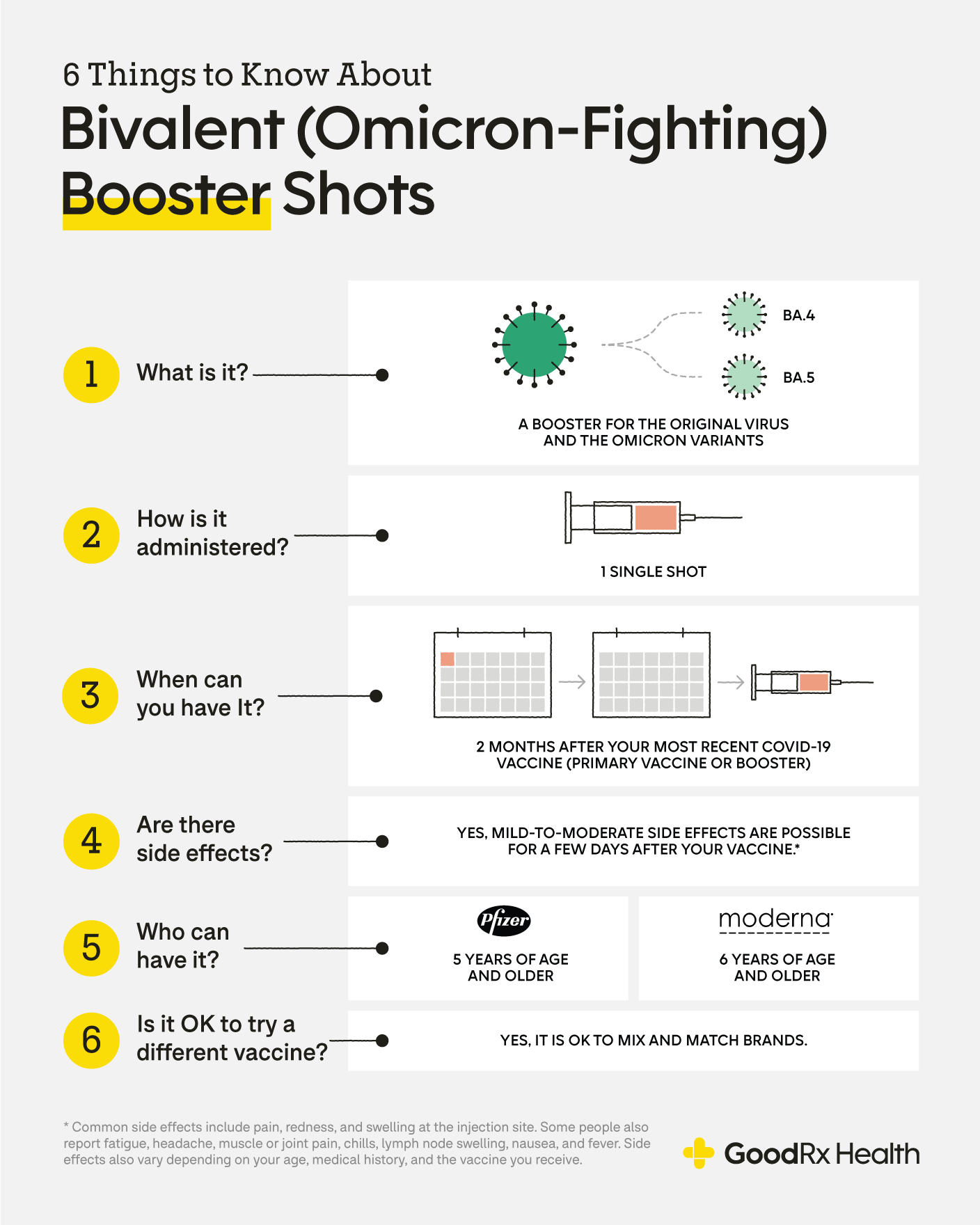
What S The Most Effective Covid 19 Booster Shot Available Today Goodrx
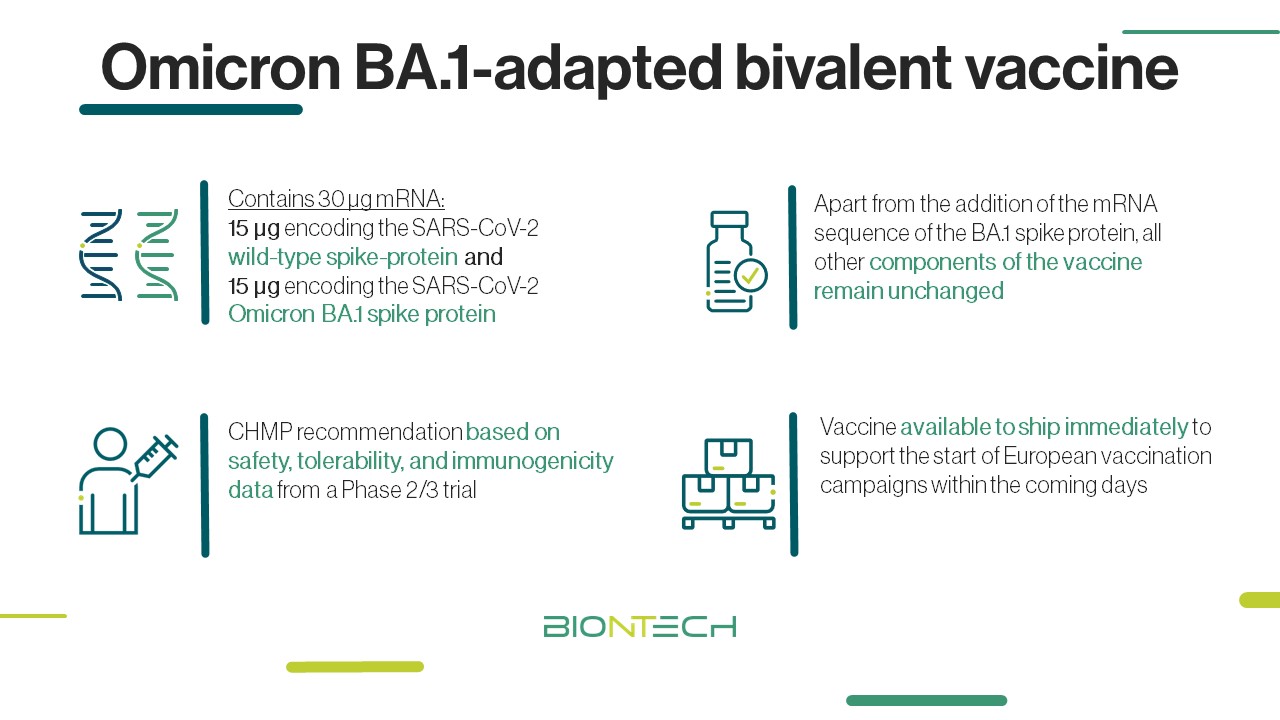
Biontech Se On Twitter Omicron Ba 1 Adapted Bivalent Vaccine Update Our Ba 1 Bivalent Covid 19 Vaccine Was Recommended For Conditional Marketing Authorization By Ema News Committee For Medicinal Products For Human Use As A Booster
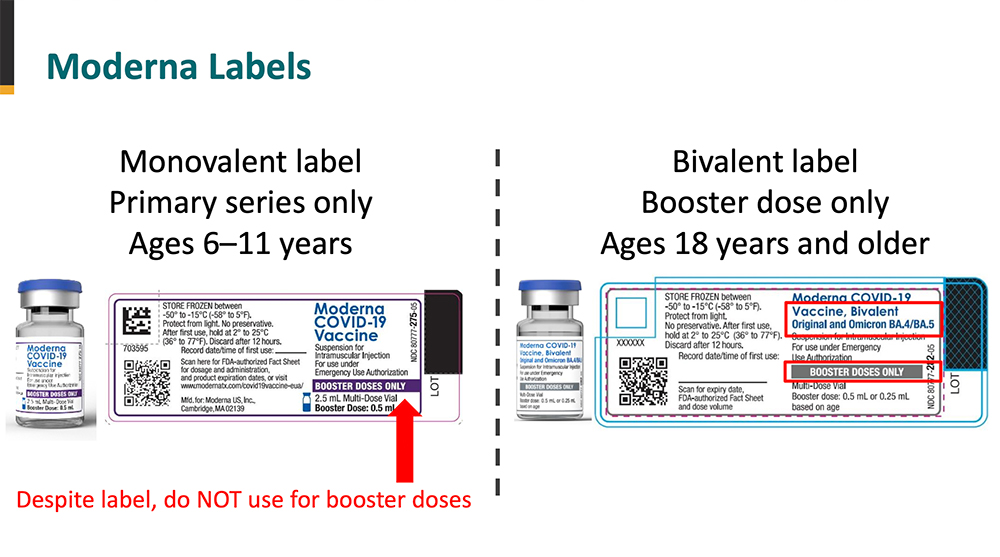
Protecting Against Covid 19 Vaccine Administration Errors Pharmacy Practice News

Bivalent Conjugate Vaccine Induces Dual Immunogenic Response That Attenuates Heroin And Fentanyl Effects In Mice Bioconjugate Chemistry

Covid 19 Bivalent Vaccine Booster Available Molokai Health Center

A Bivalent Omicron Containing Booster Vaccine Against Covid 19 Nejm
Covid 19 Bivalent Vaccine Boosters Medical Health Cluster A C

Moderna S Bivalent Booster Becomes First Authorized Omicron Vaccine

Moderna On Twitter The Government Of Canada Has Exercised Its Option To Purchase An Additional 4 5 Million Doses Of An Omicron Containing Bivalent Vaccine Booster Candidate In Addition To Moving Forward The Delivery